Electrochemical nanogravimetric study of the adsorption of 4-aminoindole and the surface layer formed by electrooxidation in aqueous acid media
The paper authored by
B.B. Berkes,
G. Inzelt and
E. Vass
is published in Electrochimica Acta (2013, vol. 96, pp. 51–60).
Abstract:
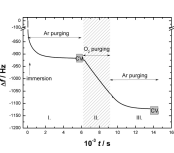
The electrochemical quartz crystal nanobalance (EQCN) was employed to study the adsorption and electropolymerization of 4-aminoindole on platinum and gold electrodes in different acidic media. It was found that a spontaneous deposition of an electrochemically active layer occurs at platinum which is accelerated in the presence of oxygen, while no such effect was observed at gold. Electrooxidation of 4-aminoindole by potential cycling up to 0.6 V leads to the formation of multilayer films at both Pt and Au electrodes. The electrogravimetric responses of these polymeric films are similar to those of the spontaneously formed adsorbed multilayer. At higher positive potential further oxidation takes place resulting in a different polymeric material which remains attached to the metal surface but shows no redox activity. The results of attenuated total reflection infrared spectroscopic (ATR-IR) and EQCN measurements attest that the amino group remains intact during the electrooxidation at lower potentials, however, due to its protonation counterions (anions) are present also in the reduced layer. The mechanism of the redox transformations of poly(4-aminoindole) involves electrochemical–chemical–electrochemical steps. The overoxidation phenomenon and stability of the polymer in different electrolytes are shown.
