Electrochemical and nanogravimetric studies of palladium phthalocyanine microcrystals
The paper authored by
Á. Nemes,
C.E. Moore and
G. Inzelt
is published in Journal of the Serbian Chemical Society (2013, vol. 78, pp. 2017–2037).
Abstract:
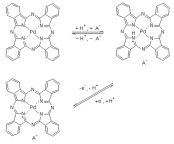
The electrochemical quartz crystal nanobalance has been used to study the redox behavior of palladium phthalocyanine microcrystals attached to gold and platinum in aqueous solutions at different pH values. In order to investigate the substrate effect, paraffin impregnated graphite electrodes were also applied. It was found that the redox transformations of palladium phthalocyanine are accompanied with deprotonation-protonation as well as the sorption and desorption of counterions, respectively, the extent of which depends on the pH. Based on the results of the nanogravimetric measurements, a redox mechanism describing also the pH dependence is suggested. Simultaneously with the charge transfer processes, solid-solid phase transitions and water transport occur.
