Microgravimetric study of electrodeposition and dissolution of lead dioxide on gold and platinum substrates
The paper authored by
B. Broda and
G. Inzelt
is published in Journal of Solid State Electrochemistry (2018, vol. 22, pp. 3921–3931).
Abstract:
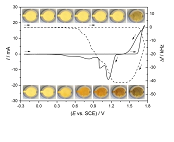
Thick lead dioxide layers were electrodeposited on gold and platinum substrates from aqueous solutions of Pb(NO3)2 dissolved in nitric acid and perchloric acid, respectively. The electrodeposition was carried out using cyclic voltammetry and chronoamperometry experiments. The mass changes during PbO2-film formation and dissolution were followed by in situ electrochemical quartz crystal microbalance (EQCM). The electrodeposition of lead dioxide on several types of substrates and in different electrolytes has been widely investigated; however, the mechanism of its dissolution has not been explored, yet. The rate of the reactions occurring during the reduction of the film can be very different depending on the substrates, the electrolytes, the applied potential, and the scan rate. The sweep rate and pH have a small effect on reversibility but highly influences the properties and the deposited mass of lead dioxide layer. At lower concentrations of nitric acid, the PbO2 can be reduced in a larger potential range which is most likely related to the variation of the conductivity of the deposited layer as well as on the nature of the intermediate species.
