Voltammetric and electrochemical impedance analysis of poly(bisphenol A) supported poly(3,4-ethylenedioxythiophene) layers deposited on gold – the effects of thickness distribution, overoxidation and non-stationarity
The paper authored by
K. Szekeres,
M. Ujvári,
S. Vesztergom and
G. G. Láng
is published in Electrochim. Acta (2021, vol. 391, 138975).
Abstract:
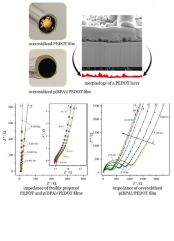
The present study describes the preparation and electrochemical characterization of poly(bisphenol A) (poly(BPA)) supported poly(3,4-ethylenedioxythiophene) (PEDOT) modified gold electrodes. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were used to characterize the electrochemistry of the Au | PEDOT | 0.1 mol/dm3 H2SO4(aq.) and Au | [poly(BPA)/PEDOT] | 0.1 mol/dm3 H2SO4(aq.) electrodes before and after overoxidation. Scanning electron microscopy (SEM) was used for the study of the structure/morphology of the polymer coatings. Similarly to overoxidized PEDOT modified electrodes, time evolution of the electrochemical properties of the poly(BPA)/PEDOT films could be observed after overoxidation. The four-dimensional (4D) analysis method (i.e., a post-experimental mathematical/analytical procedure to overcome the problems caused by non-stationarity) and complex nonlinear least-squares (CNLS) fitting has been used for the estimation of the impedance parameters corresponding to different time instants after overoxidation of the [poly(BPA)/PEDOT] film. The deviations of the impedance responses from the purely capacitive behavior predicted at low frequencies by the theoretical models could be well explained solely by the assumption of uneven film thickness. It has been found that the impedance model, which takes into account the film thickness distribution gives a good description of the impedance data, both before and after overoxidation. According to the results, after the electrochemical deposition of poly(BPA) on the PEDOT layer the resulting [poly(BPA)/PEDOT] modified electrode becomes more resistant against the negative effects of overoxidation.
