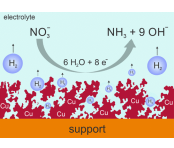
Boosting Nitrate to Ammonia Electroconversion through Hydrogen Gas Evolution over Cu-foam@mesh Catalysts
The paper authored by Y. Wang, A. Dutta, A. Iarchuk, Ch. Sun,
S. Vesztergom and P. Broekmann
is published in ACS Catalysis (2023, vol. 13, pp. 8169–8182).
Abstract:
The hydrogen evolution reaction (HER) is often considered parasitic to numerous cathodic electro-transformations of high technological interest, including but not limited to metal plating (e.g., for semiconductor processing), the CO2 reduction reaction (CO2RR), the dinitrogen → ammonia conversion (N2RR), and the nitrate reduction reaction (NO3–RR). Herein, we introduce a porous Cu foam material electrodeposited onto a mesh support through the dynamic hydrogen bubble template method as an efficient catalyst for electrochemical nitrate → ammonia conversion. To take advantage of the intrinsically high surface area of this spongy foam material, effective mass transport of the nitrate reactants from the bulk electrolyte solution into its three-dimensional porous structure is critical. At high reaction rates, NO3–RR becomes, however, readily mass transport limited because of the slow nitrate diffusion into the three-dimensional porous catalyst. Herein, we demonstrate that the gas-evolving HER can mitigate the depletion of reactants inside the 3D foam catalyst through opening an additional convective nitrate mass transport pathway provided the NO3–RR becomes already mass transport limited prior to the HER onset. This pathway is achieved through the formation and release of hydrogen bubbles facilitating electrolyte replenishment inside the foam during water/nitrate co-electrolysis. This HER-mediated transport effect “boosts” the effective limiting current of nitrate reduction, as evidenced by potentiostatic electrolyses combined with an operando video inspection of the Cu-foam@mesh catalysts under operating NO3–RR conditions. Depending on the solution pH and the nitrate concentration, NO3–RR partial current densities beyond 1 A cm–2 were achieved.
